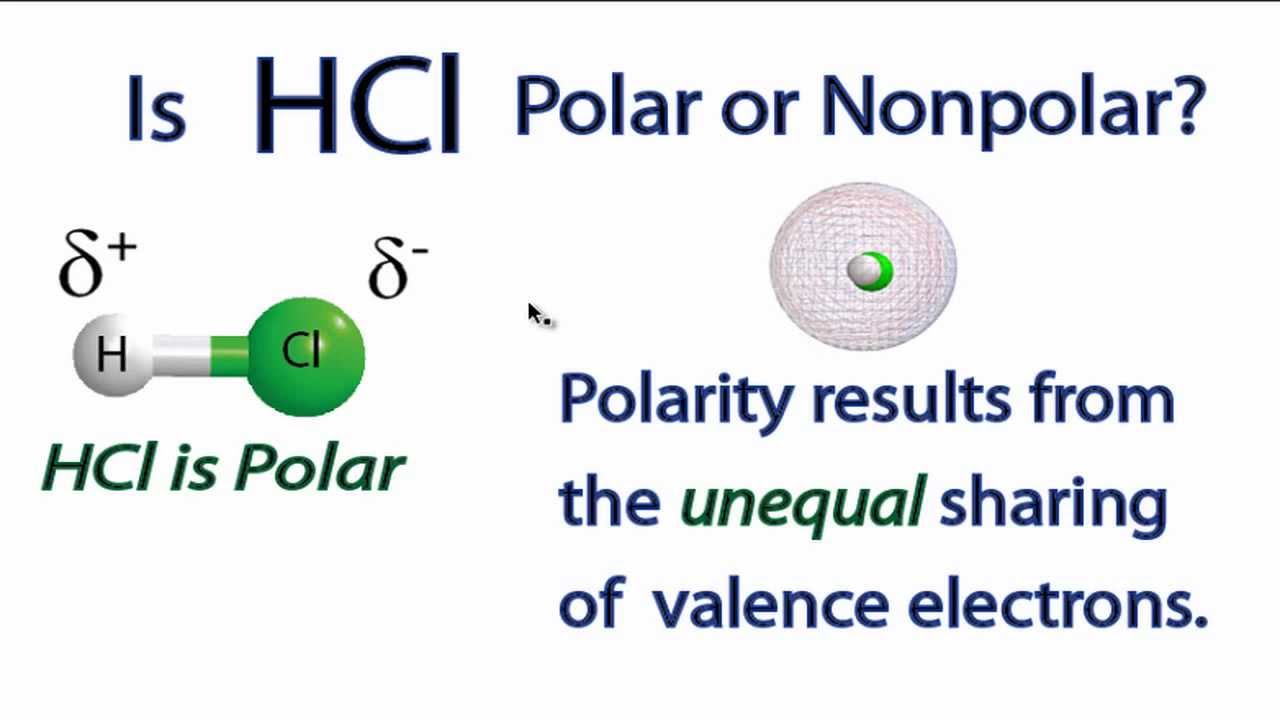
is sodium chloride polar or nonpolar
There are two reasons why dichloromethane is polar and the tetrahedral shape is only one The reasons why any molecule is polar-which is usual chemistry talk means that the molecule has a dipole moment-is that the individual dipole moments of its constituent bonds don't balance out.

PPT Molecular Geometry and Bonding Theories PowerPoint Presentation
Is CH2Cl2 Polar or Non-Polar? (Dichloromethane) Dichloromethane is a polar solvent. This means that it has a dipole moment, which is a measure of how strongly its molecules are attracted to one another. In other words, polar solvents have a positive end and a negative end, and the positive end of one molecule will be attracted to the negative.

Cis and Trans Isomers NylahropTanner
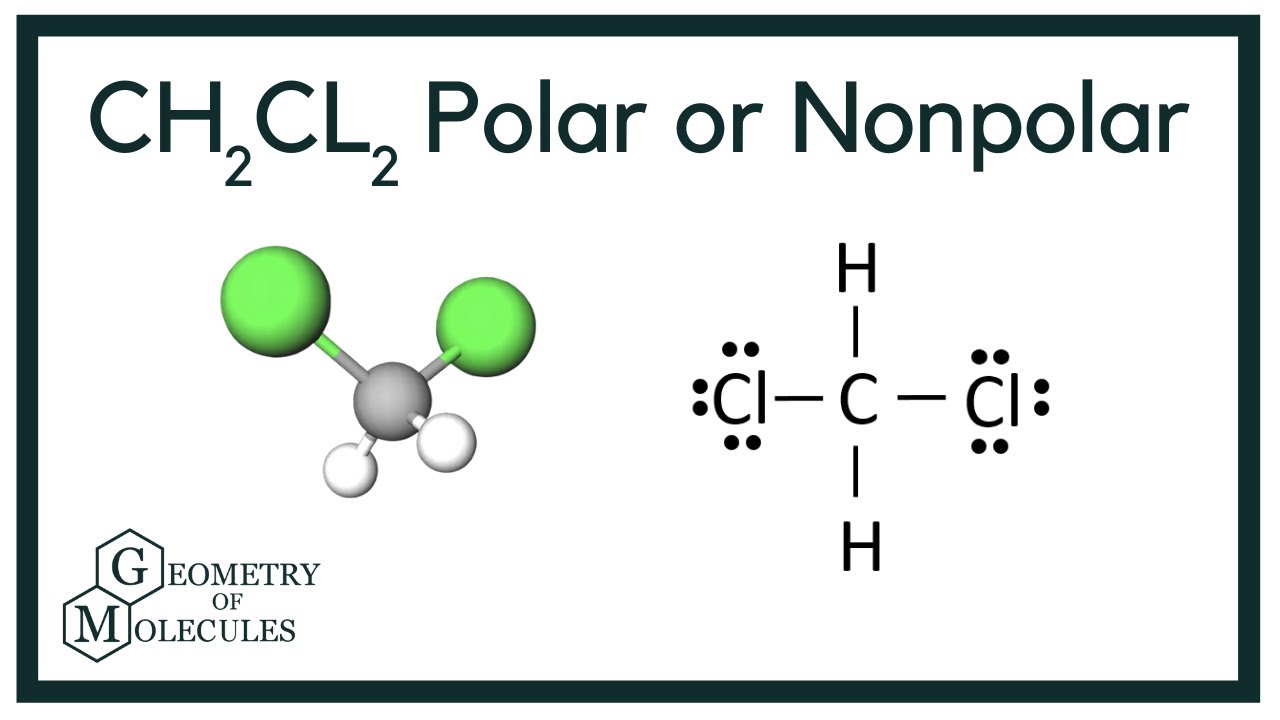
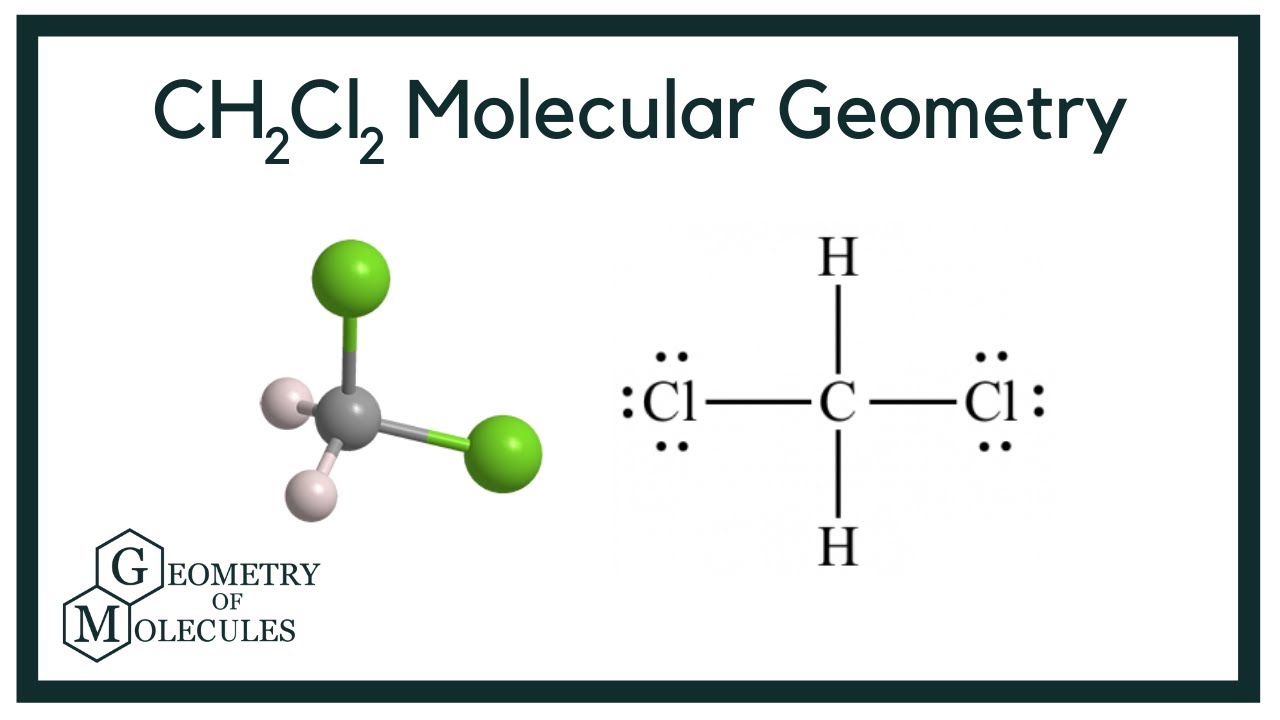
To determine if CH 2 Cl 2 (dichloromethane) is polar or nonpolar, we need to first determine its geometry. This presumes knowing the rules for drawing a correct Lewis structure and you can find more details about Lewis structures here. Carbon is the central atom: There are 4 + 2 + 2×7 = 20 electrons, and 8 have been used to make four bonds.

Brf3 Polar Or Nonpolar
Dichloromethane ( DCM, methylene chloride, or methylene bichloride) is an organochlorine compound with the formula C H 2 Cl 2. This colorless, volatile liquid with a chloroform -like, sweet odor is widely used as a solvent. Although it is not miscible with water, it is slightly polar, and miscible with many organic solvents. [12] Occurrence
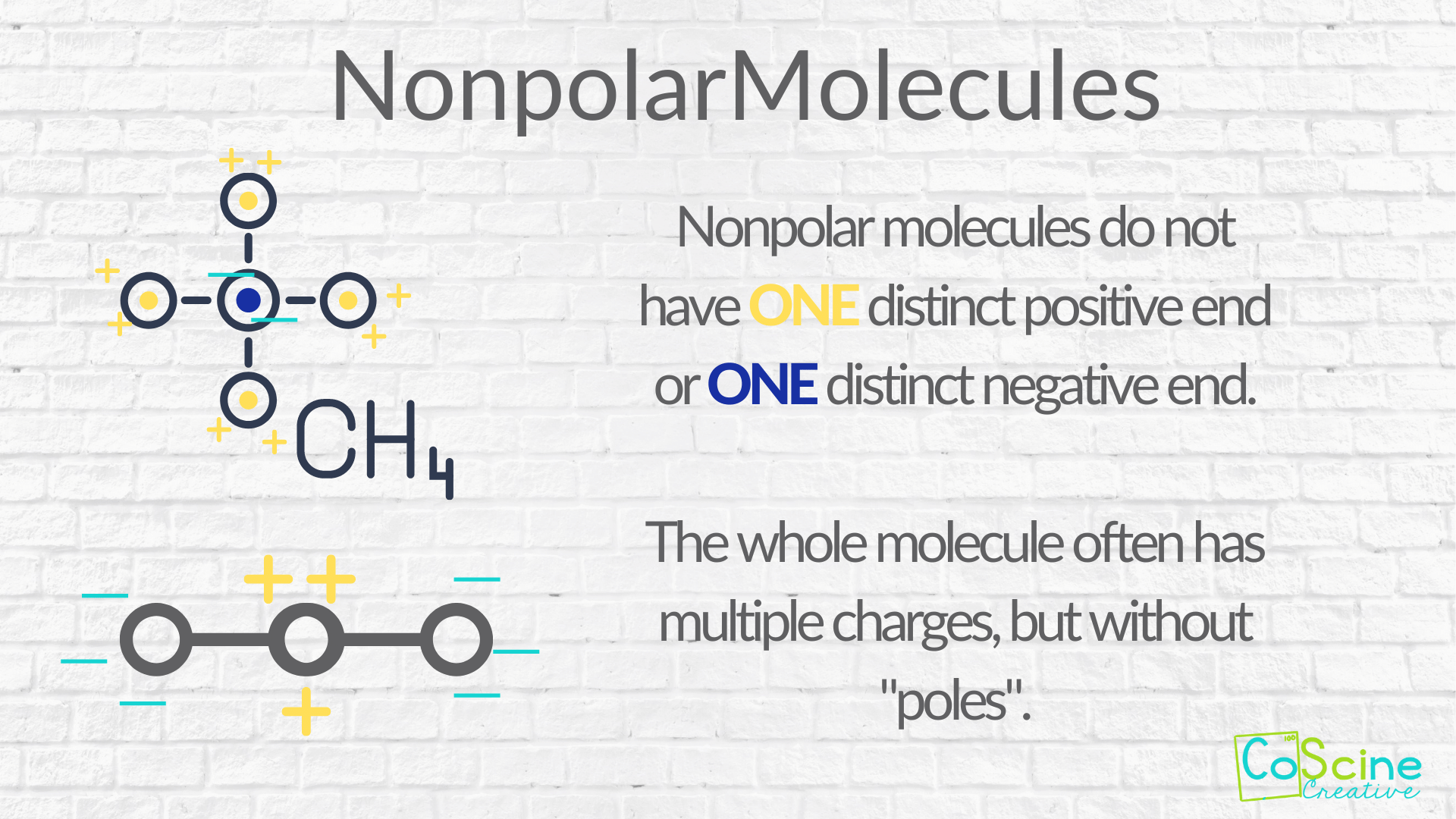
How Do You Teach Polar Vs. Nonpolar Molecules? — CoScine Creative
Dichloromethane is also polar, but it has no obvious hydrogen bond acceptor. Therefore, the most important interactions between aniline and CH 2 Cl 2 are likely to be London interactions. Water is a highly polar molecule that engages in extensive hydrogen bonding, whereas I 2 is a nonpolar molecule that cannot act as a hydrogen bond donor or.
Ch2cl2 Molecular Shape My XXX Hot Girl
Ch2Cl2 is also known as dichloromethane (or we can also say methylene chloride). It is an organic compound (molecule). In appearance, it is a liquid (colorless) and its odor is somewhat like chloroform (faint). Its observed density is 1.326 g/cm³ (at a temperature of 20 degrees Celsius) and boils at a temperature of 39.6 degrees Celsius.

Polar And Nonpolar Chart
In the compound Dichloromethane, you will find Carbon atoms with 4 electrons, and Hydrogen atoms have 2 electrons in their neutral form. The two atoms need additional electrons to complete the bond formation. Chlorine atoms, on the other hand, have 17 (seventeen) electrons distributed around their nucleus.
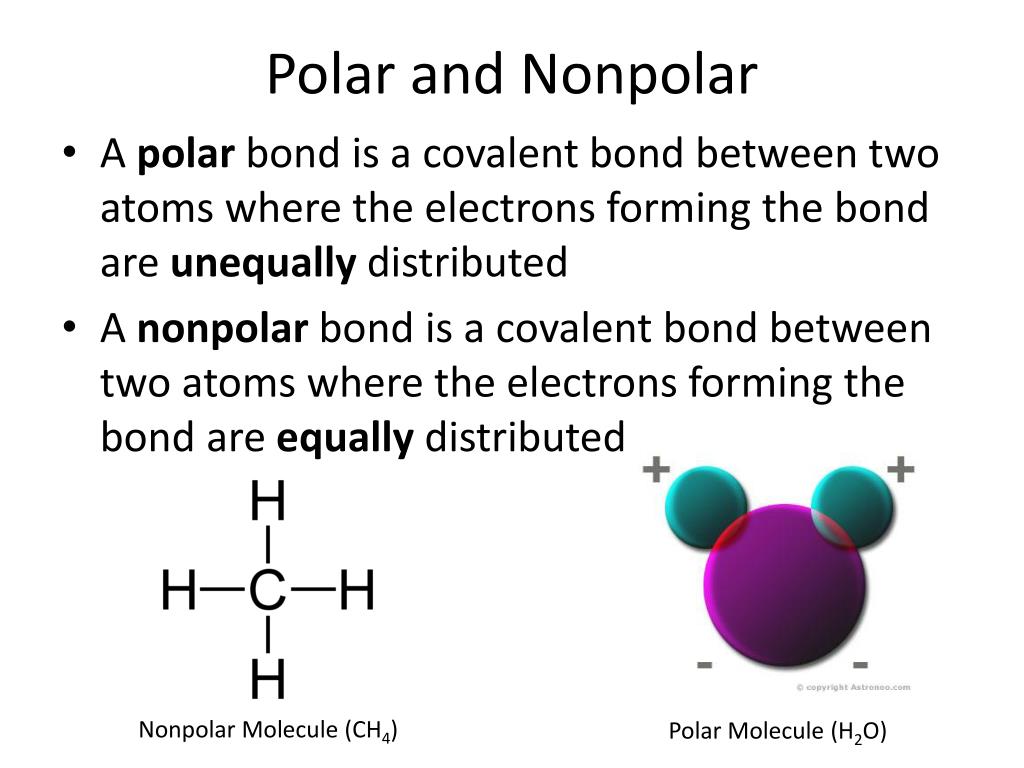
Ch4 Polar Or Nonpolar Compound What is Chemical Bonding Types of
Hey Guys!In this video, we are going to determine the polarity of Dichloromethane having a chemical formula of CH2Cl2. It is made of one Carbon atom, two Chl.

Atomic Bonds Biology for Majors I
CH2Cl2 is a polar molecule due to its tetrahedral geometrical shape and difference between the electronegativity of Carbon, Hydrogen and Chlorine atoms. This develops a dipole moment across C-Cl and C-H bonds and the entire molecule results in a net 1.67 D dipole moment. Methyl Chloride is majorly produced by the emission through industries.

O ch2cl2 é polar por que, como, quando e fatos detalhados
Learn to determine if CH2Cl2 (Dichloromethane) is polar or non-polar based on the Lewis Structure and the molecular geometry (shape).We start with the Lewis.

Is dichloromethane CH2Cl2 polar or nonpolar? Explain YouTube
This mixed solvent is mostly nonpolar due to the high percentage of hexane, but is more polar than straight hexane, due to the presence of some ethyl acetate (which has polar bonds, Figure 2.21a). The second plate was run using a 3:2 hexane:ethyl acetate mixture, which is more polar than the 6:1 mixture because there is a higher percentage of.

Ch2cl2 3d Structure
Molecules are three-dimensional, and direction is as important as magnitude when it comes to adding vectors. For example, a two-dimensional representation of the methylene chloride molecule (CH 2 Cl 2) shown below might lead to the erroneous conclusion that it is nonpolar when in fact it is polar.
CH2Cl2 Molecular Geometry, Bond Angles & Electron Geometry
In non-polar solvents like pentane and hexane, most polar compounds will not move, while non-polar compounds will travel some distance up the. Ethanol:Hexane/Pentane - 5-30% useful for very polar compounds Dichloromethane:Hexane/Pentane - 5-30% sometimes useful . 3) Fill TLC chamber . with 1-2 mL of the desired solvent system. Place a large.

Cloruro de diclorometano deuterado cloroformo deuterado, achtung
In the organic laboratory, reactions are often run in nonpolar or slightly polar solvents such as toluene (methylbenzene), hexane, dichloromethane, or diethylether. In recent years, much effort has been made to adapt reaction conditions to allow for the use of 'greener' (in other words, more environmentally friendly) solvents such as water.

Which has greater dipole moment cis1,2dichloroethylene or 1,1
Dichloromethane (DCM), also described as methylene chloride, is an organic molecule. This is generally used as a solvent in most organic reactions due to its polarity. Students generally ask question "Is CH2Cl2 polar or nonpolar?" DCM has the chemical formula CH2Cl2. It contains two hydrogen and two chlorine atoms in a tetrahedral structure.

Difference between polar and nonpolar examples
Because non-polar solvents tend to be aprotic,the focus is upon polar solvents and their structures. Solvent Polarity. Solvents are generally classified by the polarity, and considered either polar or non-polar, as indicated by the dielectric constant.. POLAR APROTIC SOLVENTS : dichloromethane, CH 2 Cl 2: 40: 9.1: tetrahydrofuran (THF), cyc.